how do leucine zippers work
The polypeptide segments containing these periodic arrays of leucine residues are thought to exist in an a-helical conformation and the leucine side chains extending from one a helix interdigitate with those. Leu-X6-Leu-X6-Leu-X6-Liu where X may be any residue.
My Masters Work -.

. A zipper zip fly or zip fastener formerly known as a clasp locker is a commonly used device for binding the edges of an opening of fabric or other flexi. C-Jun where the leucine zipper domain has become involved in transcription activation but this is the exception. How Do Zippers Work Quora Principle Of The Universal Peptide Break Technology And Leucine Zipper Download Scientific Diagram Modelling Of The Interaction Of The Atbzip63 Helical Download Scientific Diagram Dna Binding Proteins.
In the basic-region leucine-zipper domain flexible DNA-binding arms are juxtaposed by a two-stranded parallel coiled-coil motif called the leucine zipper. Basic region and the periodic arrays of leucine residues is referred to as the basic-region leucine zipper or bZIP motif. Transcription factorsaspects of Transcription.
The leucine zipper structure is adopted by one family of the coiled coil proteins. The zipper is made up of 2 main parts. A basic region that recognizes a specific DNA sequence and a series of leucines spaced 7 residues apart along an α-helix leucine zipper that mediate dimerization.
DNA Binding and Phosphorylation Regulate the Core Structure of the NF-κB p50 Transcription Factor. There are a few cases eg. It does not contain homeodomain or zinc fingers or an H-T-H motif.
Leucine repeat but do not adopt the leucine zipper structure we shall refer to these as non. Leucine Zipper domains allow subunits of a transcription factor to bind together. The leucine zipper ZIP motif consists of a periodic repetition of a leucine residue at every seventh position and forms an αhelical conformation which.
However many sequences have the leucine repeat but do not adopt the leucine zipper structure we shall refer to these as non-zippers. The leucine zipper is a dimerization domain occurring mostly in regulatory and thus in many oncogenic proteins. Leucine zippers have a characteristic leucine repeat.
How do leucine zippers work Tuesday March 15 2022 Edit Her she two been other when there all during into school time may years more most only over city some world would where later up such used many can state about national out known university united then made. In some transcription factors the leucine zipper domain extend into a new sequence-specific DNA binding domain as well. The leucine zipper is a left-handed parallel dimeric coiled-coil.
Leucine zipper domains are made up of two motifs. Genetic physical and structural studies of the leucine zipper identify interactions that help determine the stability and specificity of dimerization and DNA binding. The leucine zipper ZIP motif consists of a periodic repetition of a leucine residue at every seventh position heptad repeat and forms an αhelical conformation which facilitates dimerisation and in some cases higher oligomerisation of proteins by forming a parallel helixhelix association stabilised by formation of an interhelical hydrophobic core involving.
The B-ZIP basic-region leucine zipper class of eukaryotic transcription factors contain a leucine zipper DNA-binding motif. These motifs form a continuous α-helix that can dimerize through formation of a coiled-coil structure involving paired contacts. Leucine Zipper An Overview Sciencedirect Topics.
Leucine zippers are α-helices. What are zinc leucine zipper fingers. The leucine zipper ZIP motif consists of a periodic repetition of a leucine residue at every seventh position heptad repeat and forms an α-helical conformation which facilitates dimerisation and in some cases higher oligomerisation of proteins by forming a parallel helixhelix association stabilised by formation of an interhelical hydrophobic core involving.
Leucine Zipper - Web Books Publishing.
Structure And Mutations Of Seipin Bscl2 Schematic Illustration Of Download Scientific Diagram

Principle Of The Universal Peptide Break Technology And Leucine Zipper Download Scientific Diagram

Molecular Basis Of The Out Of Register Leucine Zipper Assembly In Mitf Download Scientific Diagram

By511 Lecture 21 Leucine Zipper Protein Motif Flashcards Quizlet
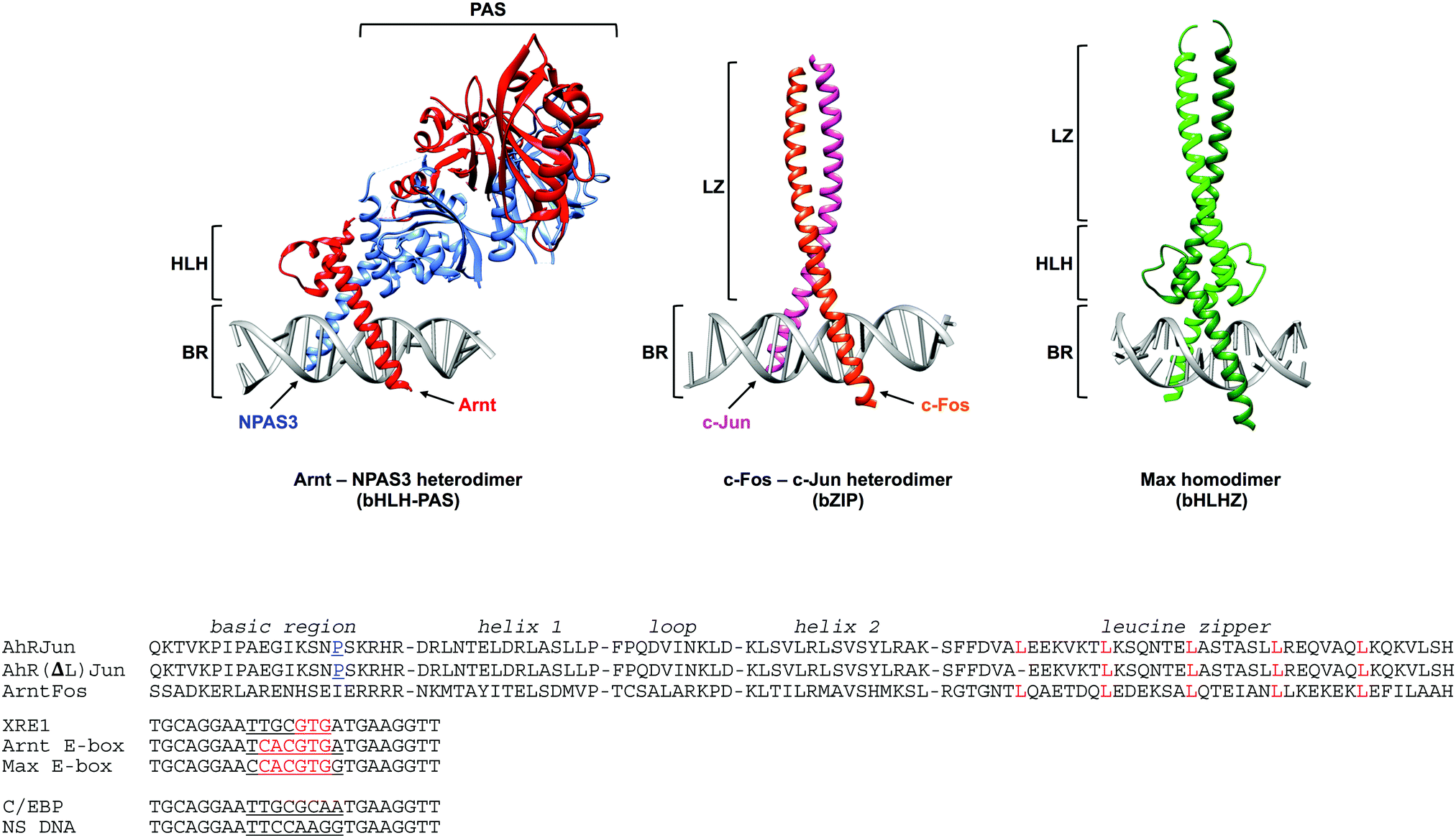
The Dna Target Determines The Dimerization Partner Selected By Bhlhz Like Hybrid Proteins Ahrjun And Arntfos Molecular Biosystems Rsc Publishing Doi 10 1039 C6mb00795c

Examples Of Protein Encapsulation Into Or Immobilization Onto Viral Download Scientific Diagram

Model Of C Myc Max And Mxd Max In Transcriptional Regulation Proteins Download Scientific Diagram

Attractive Interhelical Electrostatic Interactions In The Proline And Acidic Rich Region Par Leucine Zipper Subfamily Preclude Heterodimerization With Other Basic Leucine Zipper Subfamilies Journal Of Biological Chemistry

Leucine Zipper And The Zinc Fingers Youtube
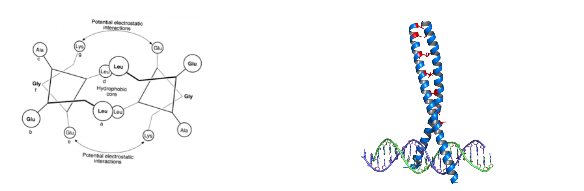
Solved Description Leucine Zipper Shown Below Is Chegg Com

A Combined Computational And Experimental Approach Reveals The Structure Of A C Ebpb Spi1 Interaction Required For Il1b Gene Transcription Journal Of Biological Chemistry
What Is The Role Of Transcription Factors In Gene Expression Quora
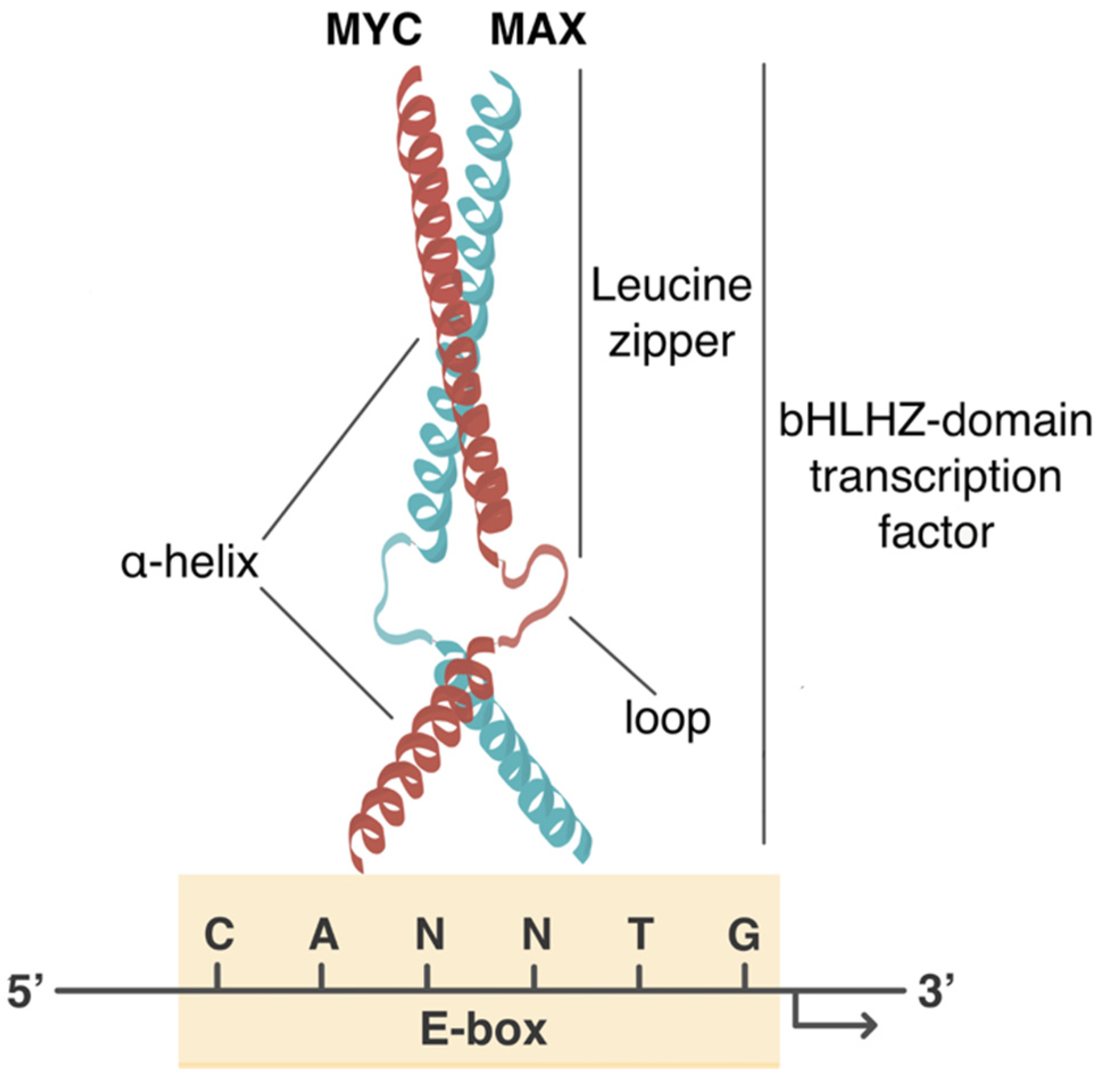
Cancers Free Full Text Myc Rules Leading Glutamine Metabolism Toward A Distinct Cancer Cell Phenotype Html
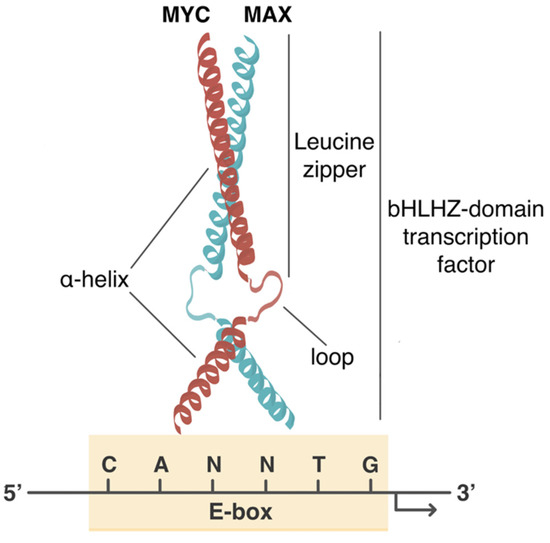
Cancers Free Full Text Myc Rules Leading Glutamine Metabolism Toward A Distinct Cancer Cell Phenotype Html

By511 Lecture 21 Leucine Zipper Protein Motif Flashcards Quizlet

X Ray Structure Of Gcn4 B Zip Dimer Bound To Double Stranded Dna 10 Download Scientific Diagram
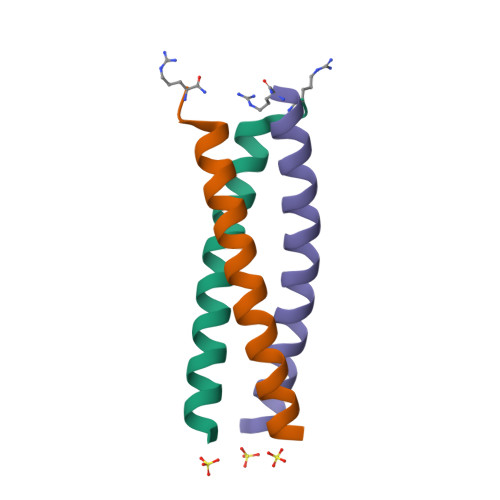
Rcsb Pdb 4dme Gcn4 Leucine Zipper Domain In A Trimeric Oligomerization State

